Through growth in the global EPA market, we will realize our mission to create a healthier, more sustainable future.
Business Overview
Since the late 1970s, Nissui has been engaged in research, production, and commercialization of EPA found in blue-backed fish such as sardines.
Through joint research on EPA with pharmaceutical companies, we established advanced purification technology and began producing and supplying EPA as pharmaceutical raw materials following approval for treatment of arteriosclerosis obliterans in 1990 and hyperlipidemia in 1994. In 2021, we obtained FDA certification. Recognizing the diverse functions of EPA, we produce and supply this compound as a functional raw material for various foods for specified health use, foods with functional claims, supplements, infant formula, nutritional products, and general foods.
Business Strengths
- Fish oil procurement capability utilizing our global network
- EPA purification technology and production capabilities, world-class unrefined fish oil storage facilities
- Technology to deodorize fish oil, which is prone to oxidation and odor, to near odorless levels
- Technology to suppress fish odor when adding EPA and DHA to food products
- Track record of supplying pharmaceutical raw materials for over 40 years
Business Areas
Pharmaceutical Raw Materials
- In 1980, we successfully established high purity EPA technology and became the world's first company to manufacture EPA as a pharmaceutical raw material. For over 40 years, we have maintained a consistent supply of raw materials for EPA pharmaceutical formulations.
Functional Raw Materials
- We produce and supply high-quality EPA as a functional raw material to manufacturers of supplements, infant formula, nutritional products, and general foods.
Functional Foods
- We offer drinks, powders, and gummies incorporating functional materials such as EPA and DHA.
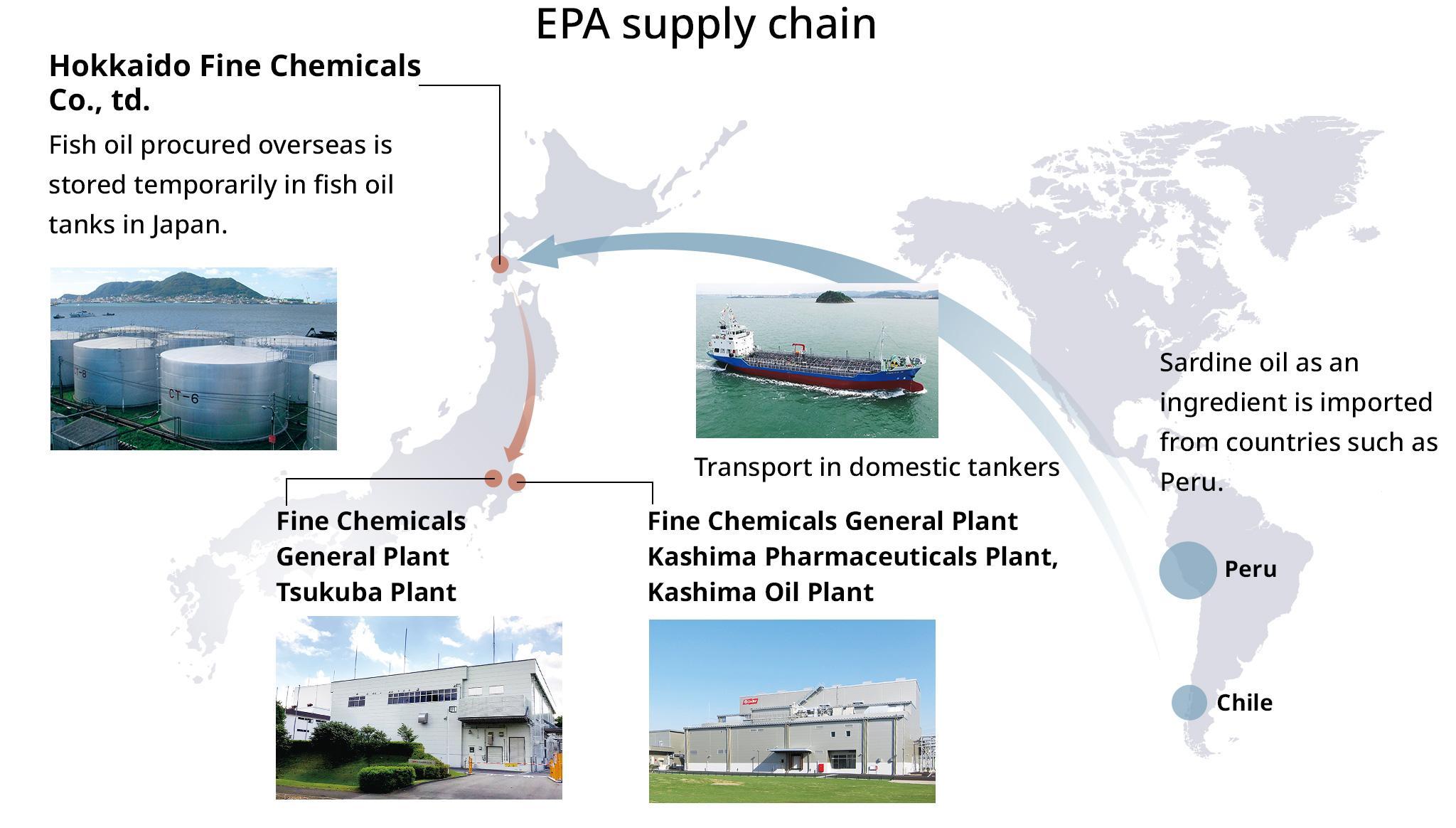
EPA Supply Chain
The Nissui Group procures sardines, processes and stores sardine oil, and produces EPA at the Nissui Group plants in cooperation with Group companies in Japan and overseas. For raw material sardine oil, we import from countries such as Peru and also use domestically produced sardine oil. Based on our unique EPA supply chain and technical capabilities developed through years of research, we deliver high-value-added products worldwide.